Introduction
With both parts of my bioprocessing primer out of the way, and enough news flow to make any company a battleground stock, it’s time to talk about Catalent.
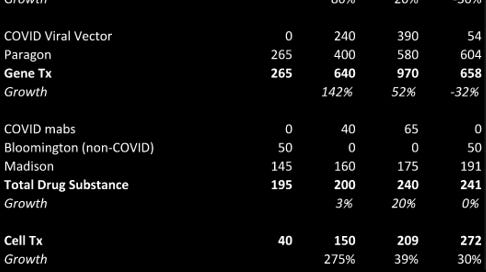
Catalent is a CDMO that has evolved a lot since it went public around a decade ago. From 2013-2019, the company grew ~6% annualized, which at the surface is rather unimpressive. However, …